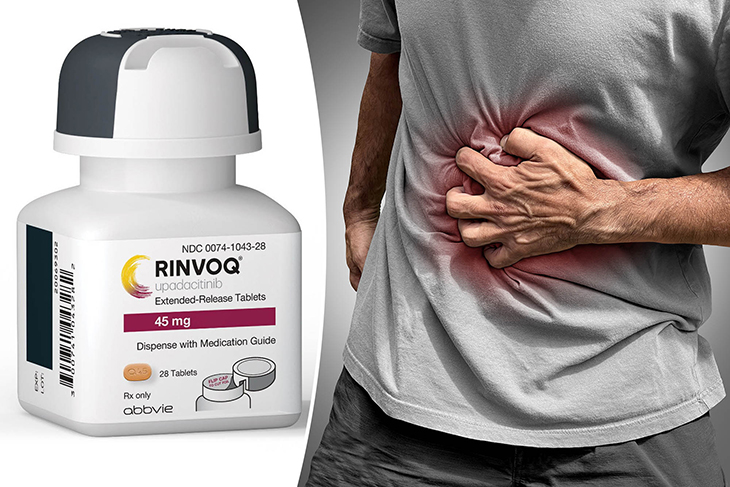
Rinvoq has the capacity to diminish inflammation and ameliorate symptoms in individuals dealing with moderate to severe Crohn’s disease. Available in tablet form, Rinvoq, an inhibitor of JAK enzymes (upadacitinib), presents a novel and rapid-acting option as an alternative to infusion and injectable therapies.
On May 18, the U.S. Food and Drug Administration (FDA) provided its approval for Rinvoq (upadacitinib) to address the needs of adults afflicted by moderately to severely active Crohn’s disease, especially those who have not shown favorable responses to, or cannot tolerate, one or more tumor necrosis factor (TNF) blockers.
With this FDA announcement, Rinvoq emerges as the pioneering oral medication sanctioned for the treatment of moderate to severe Crohn’s disease. AbbVie, the manufacturer of Rinvoq, revealed in a press release that the drug has already secured approval for managing ulcerative colitis and several other conditions.
“This approval is very important, as it now gives our patients the option to choose an oral therapy for their Crohn’s disease,” said Farah Monzur, MD. She’s an assistant professor of medicine, a gastroenterologist, and the director of the inflammatory bowel disease (IBD) center at Stony Brook Medicine in New York. It also must be noted that Dr. Monzur was not part of the development of Rinvoq or its clinical trials.
“Previously, the treatment options for people with moderate to severe Crohn’s disease have been limited to intravenous (IV) infusions, injectables, and non-FDA approved oral medications,” Monzur added.
A JAK Inhibitor That Can Work Quickly for Symptom Control
According to information from MedlinePlus, over 500,000 individuals in the United States suffer from Crohn’s disease, a persistent inflammatory condition affecting the digestive tract. This ailment leads to inflammation throughout the digestive tract, extending from the mouth to the anus, although it predominantly occurs in the initial segments of the large intestine and the small intestine.
The symptoms of Crohn’s disease can differ based on the intensity and location of the inflammation. Typical indications encompass diarrhea, abdominal discomfort, spasms, and loss of weight.
In dealing with Crohn’s disease, treatment pursues two primary objectives: firstly, achieving a state of remission where inflammation and symptoms are controlled; subsequently, upholding this remission, as outlined by the Mayo Clinic. Numerous classes of medications are deployed to manage this disease, including immunomodulators, biologics, and small molecules, which comprise substances like Janus kinase (JAK) inhibitors and S1P receptor modulators.
Among these, Rinvoq constitutes a form of JAK inhibitor that functions by obstructing various pathways responsible for inflammation provoked by inflammatory bowel diseases. Distinct from certain orally ingested medications like thiopurines (6-mercaptourine and azathioprine) and methotrexate—requiring several weeks to regulate inflammation—JAK inhibitors work with greater rapidity to attain and sustain remission, as indicated by the Crohn’s & Colitis Foundation.
First Oral Treatment Approved for Use for Moderate to Severe Cases
Two clinical trials were conducted to assess the safety and effectiveness of Rinvoq in individuals with moderate to severe Crohn’s disease, totaling 857 participants. These participants were randomly divided into two groups: one receiving a daily dose of 45 milligrams (mg) of Rinvoq, and the other receiving a placebo, both administered over a period of 12 weeks.
At the conclusion of the 12-week period, a greater proportion of patients who received Rinvoq achieved clinical remission and exhibited improvements in intestinal inflammation compared to those who were given the placebo. This evaluation was based on assessments using the Crohn’s Disease Activity Index (CDAI) and colonoscopy examinations.
The Crohn’s Disease Activity Index (CDAI) is widely recognized as the definitive standard for evaluating Crohn’s disease in clinical trials. It monitors various factors such as stool frequency, abdominal pain, overall well-being, and usage of antidiarrheal medications in the preceding week.
Rinvoq – Effective as a Maintenance Therapy
To evaluate Rinvoq’s effectiveness as an ongoing treatment, a study was conducted involving 343 individuals who had shown positive responses to a 12-week regimen of 45 mg of Rinvoq taken once daily. Following this, the participants were randomly assigned again to receive a maintenance routine of either 15 mg or 30 mg of Rinvoq taken once daily, or they were given a placebo, all for a duration of 52 weeks. This added up to a minimum total therapy duration of 64 weeks.
Upon completing the 52-week period, a greater proportion of patients who received either 15 mg or 30 mg of Rinvoq achieved clinical remission and exhibited improvements in intestinal inflammation when compared to those who were on the placebo. These assessments were based on the evaluations of the CDAI scores and colonoscopy results.
“Symptoms of moderately to severely active Crohn’s disease can be disruptive and uncomfortable for patients, so relief as early as possible is key. Given the progressive nature of the disease, endoscopic response [visible reduction of intestinal lining damage] is just as important,” said Edward V. Loftus Jr., MD, when he gave a press release. He is a professor of medicine in the division of gastroenterology and hepatology at Mayo Clinic in Rochester, Minnesota as well as an investigator for the clinical trials.
“Based on the clinical trial results, treatment with Rinvoq shows both early and long-term symptom relief along with evidence of a visible reduction of damage to the intestinal lining caused by excess inflammation,” Dr. Loftus said.
Rinvoq – Its Efficacy and Safety
Rinvoq may lead to adverse reactions such as upper respiratory tract infections, anemia, fever, acne, herpes zoster (shingles), and headaches.
Combining Rinvoq with other JAK inhibitors, biological treatments for Crohn’s disease, or potent immunosuppressants like azathioprine and cyclosporine is not recommended.
JAK inhibitors, including Rinvoq, have been associated with severe infections, mortality, malignancies, significant cardiovascular events, and thrombosis. Consequently, the drug carries a black box warning.
Those Who May Benefit from Rinvoq
Rinvoq has been approved only for those who have had an inadequate response or intolerance to one or more TNF blockers, Monzur stressed. “Patients who would benefit the most from Rinvoq would be those who have not had a response from TNF blockers and need a rapid clinical response. The improvement of clinical symptoms can be as early as two weeks from the start of the medication,” she also said.
“I would not recommend Rinvoq to patients who are over 50 years old with at least one heart disease risk factor, current or past smokers, those with active cancer or those with a risk of forming blood clots. I would be cautious in prescribing to pregnant patients,” Monzur said.
Is Rinvoq the Best Treatment for You? Talk With Your Gastroenterologist
Right now, it’s not yet known if Rinvoq reigns superior to existing Crohn’s disease treatments, Monzur explained. “As with studies on other IBD medications, the studies done on Rinvoq compared it to placebo, not to the list of available Crohn’s disease medications we currently have. Thus, it is difficult to compare one IBD medication to another.”
There are several medications as well, all with a variety of side effects, she stated. “I would urge patients to see gastroenterologists who specialize in IBD so they can have a discussion on the right medication for them. It is important to have shared decision-making between physicians and patients,” she advised.