The U.S. Food and Drug Administration (FDA) has granted approval for the first medication aimed at mitigating severe allergic reactions stemming from accidental exposure to specific foods.
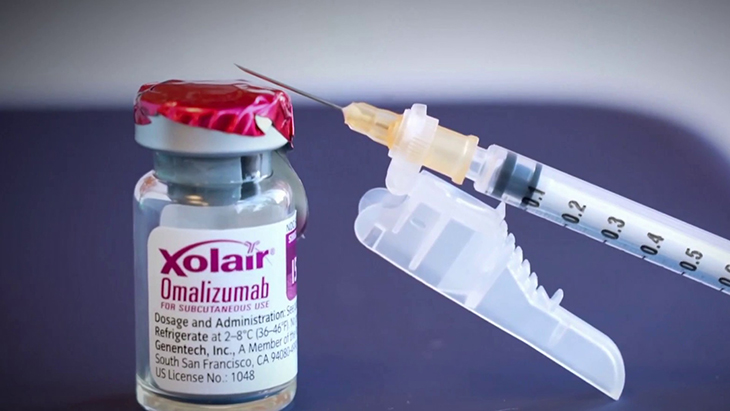
Xolair (omalizumab), and injectable drug initially sanctioned for asthma treatment two decades ago, has now received clearance to combat severe and potentially life-threatening allergic reactions triggered by common allergens such as peanuts, milk, eggs, and wheat, as disclosed by the FDA. This medication is suitable for addressing allergies to multiple foods in both adults and children aged one year and above.
“This newly approved use for Xolair will provide a treatment option to reduce the risk of harmful allergic reactions among certain patients,” said Kelly Stone, MD, PhD, the associate director of the division of pulmonology, allergy, and critical care in the FDA’s Center for Drug Evaluation and Research, in a statement.
“While it will not eliminate food allergies or allow patients to consume food allergens freely, its repeated use will help reduce the health impact if accidental exposure occurs.”
According to the FDA, more than 1 in 20 individuals in the United States suffer from allergies to at least one food capable of eliciting severe and potentially life-threatening allergic responses.
The Risk of Anaphylaxis Posed by Food Allergies
The risk of anaphylaxis looms large with such allergies, posing a grave concern. Anaphylaxis can swiftly ensue within seconds of exposure to specific foods, manifesting symptoms like rash, nausea, vomiting, and respiratory distress. Without prompt treatment – typically administered via an EpiPen containing epinephrine – anaphylaxis can lead to unconsciousness and even fatalities.
“Xolair is a preventive treatment that can reduce the risk of allergic reactions in response to accidental ingestion of allergens, if used prior to allergen exposure,” says Sayantani Sindher, MD, a clinical associate professor of medicine at Stanford University in Palo Alto, California. “In contrast, rescue medications such as EpiPens are used after an allergic reaction is triggered and act to relieve the symptoms of allergic anaphylactic reactions.”
Individuals afflicted with food allergies are typically counseled to adhere to a stringent dietary regimen, steering clear of any exposure to allergenic foods known to provoke severe allergic responses. Moreover, carrying epinephrine for emergency use is strongly recommended. Notably, the only alternative medication sanctioned for food allergies, Palforzia, is exclusively prescribed for peanut allergies in children aged 4 to 17.
New Medication Can Prevent Multiple Food Allergies
Xolair heralds a groundbreaking advancement in allergy treatment, being the first drug endorsed to address multiple food allergies simultaneously, as stated by the FDA.
Clinical trials have yielded promising results, with 68 percent of patients administered Xolair for peanut allergies demonstrating the ability to consume up to 600 milligrams of peanut protein without experiencing moderate or severe allergic reactions. Comparable outcomes were observed across other food allergens such as cashews, milk, and eggs.
However, efficacy varied among individuals, with approximately 17 percent of participants in clinical trials reporting minimal improvement in peanut tolerance despite Xolair injections. Common side effects associated with Xolair include reactions at the injection site and fever.
Moreover, the FDA has cautioned about the potential risk of anaphylaxis, necessitating initial administration under medical supervision to mitigate adverse reactions.
Dr. Zahida Maskatia, the medical director at Latitude Food Allergy Care, highlights the importance of continued Xolair treatment to safeguard against severe allergic reactions triggered by food allergens. Additionally, she advises individuals to maintain access to EpiPens for prompt intervention in emergencies.
“It is important to know that Xolair does not work immediately and should not be used to treat an allergic reaction,” Dr. Maskatia says.
Dr. Maskatia explains that epinephrine, in contrast, acts rapidly to address severe allergic reactions, including anaphylaxis. This swift action in attributed to its ability to swiftly alleviate throat swelling, facilitate airway opening, and uphold heart function and blood pressure.
Dr. Maskatia says, “Patients who are on Xolair may be safer, but since this is not a cure, they will still need to avoid their food allergens, and, importantly, they must always carry epinephrine.”