FDA Finally Gave Its Approval For Spinal Cord Simulators, Helping Those Who Suffer From Chronic Back Pain
Roughly 50 million Americans are grappling with persistent back pain, as per various estimations. While trials have indicated noteworthy decreases in pain, some specialists suggest that more extensive and longer-term investigations are imperative to validate these advantages.
In an announcement issued by Abbott, a pharmaceutical company, on May 16, 2023, it was revealed that the U.S. Food and Drug Administration (FDA) has sanctioned the use of spinal cord stimulation (SCS) devices for treating chronic back pain in individuals who either have not undergone back surgery or are ineligible for it. The FDA’s approval followed a six-month study involving 200 participants, where Abbott’s spinal cord stimulation showcased substantial relief and enhancements in pain, functionality, quality of life, and psychological well-being.
The individuals partaking in the study had been grappling with incapacitating chronic back pain for nearly 13 years on average. These participants were not suitable for corrective back surgeries due to being medically fragile or having multiple degenerative spinal changes that did not meet the severity criteria for corrective surgery but were still causing significant symptoms.
After a period of six months, it was observed that approximately 85 percent of those who had received Abbott’s SCS devices achieved noteworthy reductions in back pain, a stark contrast to the mere 7 percent who underwent conservative medical management. On average, individuals undergoing SCS therapy experienced a pain reduction of around 70 percent.
“Historically, people who have no options for corrective surgery to address their chronic back pain are usually treated with combinations of therapies: physical therapy and chiropractic care, injections, and pain relievers,” Allen Burton, MD, the divisional vice president and chief medical officer of Abbott’s neuromodulation business, said. “However, these options are not effective for a significant subset of people who previously did not have other therapies available, while causing the treatment journey to feel complicated and uncertain for others. People who suffer from chronic back pain — and do not fit the typical surgical criteria — tend to forgo future treatment, ultimately resign themselves to living with debilitating chronic pain.”
According to the U.S. Pain Foundation, approximately 50 million individuals in the United States are grappling with persistent back pain.
This enduring discomfort often arises due to conditions like arthritis, spinal stenosis, or issues with spinal discs. Nonetheless, identifying the precise source of chronic back pain can pose challenges, thereby complicating the creation of effective treatment strategies. Dr. Burton points out that medical professionals might advise the utilization of more cautious methodologies in addressing chronic back pain, such as engaging in exercises, undergoing physical therapy or chiropractic sessions, utilizing pharmaceutical interventions, and considering injections. Nevertheless, even when these more conservative techniques are combined, they might not always furnish the desired relief from persistent pain for certain individuals.
Advancing a Former Concept
Although the application of electrical stimulation for pain relief might appear contemporary, studies suggest that this concept traces its origins to ancient Rome. During that time, the physician Scribonius Largus discovered that gout pain could be alleviated through contact with the torpedo fish, also recognized as the electric ray. This remarkable aquatic creature possesses a natural electrical charge capable of stunning other animals.
However, it was only in 1968 that the Medtronic company brought forth the initial spinal cord stimulator ready for commercial use.
Abbott’s most recent SCS devices employ their exclusive BurstDR stimulation technology, which pioneers the utilization of gentle electrical pulses to imitate the body’s natural electrical transmissions. This process conceals pain-signaling pulses en route to the brain.
Dr. Michael Leong, a pain management expert at the Stanford Cancer Center in California, unaffiliated with Abbott’s research, highlights the distinction of these pulse systems from earlier spinal stimulation technologies. Notably, patients do not perceive the sensation of stimulation.
“Other previous systems used something called ‘tonic stimulation,’ which created a tingling that patients could feel,” Dr. Leong said. “With these newer waveforms, you can’t feel the sensation.”
Leong also mentioned that substantial alleviation of physical pain, facilitated by this form of stimulation, can contribute to the reduction of emotional symptoms as well.
“A lot of people with chronic pain develop symptoms of depression and anxiety,” he says. “If a device can ease not just physical pain but the emotional pain that goes along with it, that’s a huge benefit.”
As mental and physical health improve, so do quality of life and productivity.
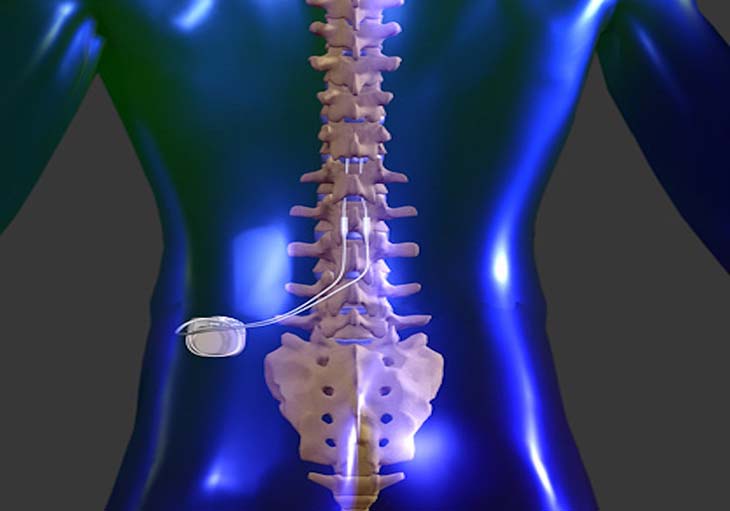
Implanting the Device
Individuals experiencing chronic back pain who have an inclination towards it, can initially test the technology for a duration of 7 to 10 days. If they perceive it as advantageous, the option to have the device implanted becomes available. Drawing a parallel, Dr. Burton likens the implantation procedure to that of a heart pacemaker, as it remains within the patient to provide ongoing pain relief.
Leong highlights that the procedure is minimally invasive in nature.
“The devices are very, very tiny,” he explained. “They used to be the size of a hockey puck, but now they’re the size of batteries in our iPhones.”
Frequently, the device is placed right below the skin on the lower back or buttock area. Delicate wires stemming from the device are carefully positioned along the spinal column, close to the nerves responsible for transmitting pain signals. The electronic components emit uninterrupted electrical pulses, effectively interfering with the pain signals’ journey to the brain.
The usual duration for implanting an SCS device ranges from one to two hours. These surgeries are commonly conducted as outpatient procedures or with an overnight stay.
Potential Limitations for the Stimulation
The most recent endorsement from the FDA for Abbott encompasses their entire range of devices currently offered within the United States. This includes their Proclaim Plus implant known for its decade-long battery life, as well as their rechargeable Eterna system.
Despite the swift acquisition of federal approvals for these devices, Dr. Mark Queralt, MD, who serves as the director of the Musculoskeletal Institute and holds the position of clinical director for back and neck pain at Dell Medical School, University of Texas in Austin, advises consumers to exercise caution. He emphasizes the importance of thoroughly evaluating all available evidence concerning these stimulators.
According to Dr. Queralt, the latest findings presented by Abbott have limitations due to their reliance on the responses of a mere 200 individuals. To truly gauge the effectiveness of this technology in a real-world context, he suggests that results from a sample size of 2,000 patients or more would be considerably more informative.
Dr. Queralt raises doubts about the efficacy of this therapy category, drawing attention to research that suggests spinal cord stimulation does not lead to a reduction in opioid use, pain injections, or spinal surgeries. Additionally, he underscores concerns about the financial aspect of such treatment. Industry analysis indicate that opting for this therapy can incur costs exceeding conventional medical therapy by $39,000 within the first year.
“People who have had chronic pain for years are desperate for anything. Hope is a powerful motivator, and this gives them hope,” he added. “I’m not overly optimistic about this technology. Look at all the evidence. Time will tell on this.”
Leong recommended that those with chronic back pain must “in good conscience” try more conservative approaches like physical therapy and medications before going for something this drastic. “But if these don’t work, and if you don’t think you’re going to have some kind of, or need, corrective surgery, this can be a great option,” he also said.



