FDA Approves Overdose Drug Narcan For Over-the-Counter Use, Expanding Access To Life-Saving Drug

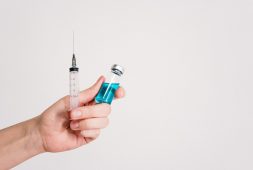
In a groundbreaking decision, the U.S. Food and Drug Administration (FDA) granted approval for Narcan, a crucial medication, to be available over the counter without a prescription. Narcan, a nasal spray containing 4 milligrams of naloxone hydrochloride, is renowned for its ability to swiftly reverse the effects of opioid overdose, making it the go-to treatment for such emergencies.
This significant move by the FDA opens the door for individuals to purchase life-saving doses directly from various retail locations, including drug stores, convenience stores, grocery stores, and gas stations, as well as through online platforms.
However, the exact timeline for when the medication will become available and its pricing will be determined by Emergent BioSolutions, the American manufacturer. The FDA has cautioned that the transition from prescription to over-the-counter status “may take several months” to complete.
It’s important to note that while Narcan will now be accessible without a prescription, other formulations and dosages of naloxone will continue to be exclusively available by prescription.
The approval of Narcan for over-the-counter use comes at a critical time, as drug overdose remains a pressing public health crisis in the United States. Disturbingly, more than 101,750 fatal overdoses were reported within the 12-month period ending in October 2022. The primary driver behind these tragic incidents is the presence of synthetic opioids, particularly illicit fentanyl.
By expanding access to Narcan and making it readily available to the public, the FDA aims to combat the escalating overdose crisis and potentially save countless lives.
FDA Commissioner Robert M. Califf, M.D. said, “The FDA has used its regulatory authority to facilitate greater access to naloxone…to address the dire public health need. Today’s approval of OTC naloxone nasal spray will increase the number of locations where it’s available and help reduce opioid overdose deaths throughout the country. We encourage the manufacturer to make accessibility to the product a priority by making it available as soon as possible and at an affordable price.”
In 2015, the FDA initially granted approval to Narcan nasal spray as a prescription medication, and since its public launch in 2016, more than 44 million doses have been distributed. As part of the process to transition the drug from prescription to nonprescription status, the manufacturer provided compelling data demonstrating the drug’s safety and efficacy when used as directed.
Additionally, the manufacturer demonstrated that consumers can comprehend how to use the drug safely and effectively without the supervision of a healthcare professional. Following this, in February 2023, the FDA committee members unanimously voted to recommend its approval for nonprescription marketing.
To accommodate the approval of over-the-counter Narcan nasal spray, changes will be required in the labeling of the currently approved 4 mg generic naloxone nasal spray products that rely on Narcan as their reference listed drug. As stated in the FDA press release, manufacturers of these products, which are priced at around $45 for two doses compared to Narcan at $140, will need to submit supplemental applications to facilitate the effective transition of their products to over-the-counter status.
It is also worth noting that the approval may impact the status of other brand-name naloxone nasal spray products containing 4 mg or less. However, determinations regarding these products will be made on a case-by-case basis, and the FDA may reach out to other companies as necessary.
Director of the FDA’s Center for Drug Evaluation and Research, Patrizia Cavazzoni, M.D., said, “Naloxone is a critical tool in addressing opioid overdoses and today’s approval underscores the extensive efforts the agency has undertaken to combat the overdose crisis. The FDA is working with our federal partners to help ensure continued access to all forms of naloxone during the transition of this product from prescription status to nonprescription/OTC status.”
He added, “Further, we will work with any sponsor seeking to market a nonprescription naloxone product, including through an Rx to OTC switch, and encourage manufacturers to contact the agency as early as possible to initiate discussions.”