In the realm of medical breakthroughs, triumphs often come in various forms, not always grandiose but nonetheless significant. While it may not be hailed as a cure for ailments like ALS or cancer, the recent development of a “game-changer” treatment for severe frostbite by pharmacists has garnered well-deserved accolades.
On February 14th, a notable milestone was achieved as the FDA granted approval for Aurlumyn (iloprost) injection, marking a pivotal moment in the realm of frostbite management in adults.
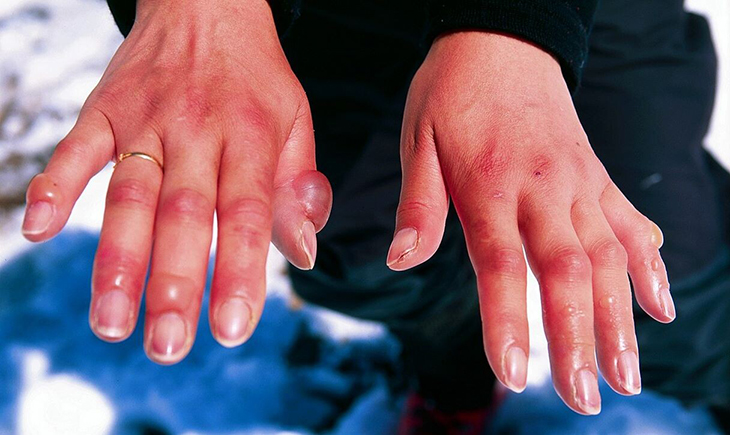
Frostbite, a condition characterized by the freezing of both skin and underlying tissue, poses grave risks, with severe cases potentially leading to the amputation of affected fingers or toes. The approval of Aurlumyn represents a beacon of hope for individuals facing the debilitating consequences of this condition.
At the heart of Aurlumyn lies iloprost, an active ingredient renowned for its vasodilatory properties, which effectively opens blood vessels and prevents clotting, thereby facilitating improved blood flow to frostbitten extremities.
Norman Stockbridge, M.D., Ph.D., director of the Division of Cardiology and Nephrology in the FDA’s Center for Drug Evaluation and Research said, “This approval provides patients with the first-ever treatment option for severe frostbite. Having this new option provides physicians with a tool that will help prevent the life-changing amputation of one’s frostbitten fingers or toes.”
The harrowing nature of frostbite is often likened to a hostage situation, where conventional methods of relief are rendered ineffective due to the delicate balance of thawing frozen tissues. Rapid warming, although intuitive, poses significant risks, potentially exacerbating tissue damage and triggering internal bleeding. The conventional wisdom of immersing frostbitten areas in hot water is full of danger, as the sudden temperature change may lead to the rupture of ice crystals within tissues, causing further harm.
One of the greatest challenges in treating frostbite lies in the restoration of blood flow to affected areas, a task complicated by the presence of frozen blood and tissue. Traditional clot-busting medications, while effective in some scenarios, carry substantial risks of inducing internal hemorrhaging, underscoring the urgent need for safer and more efficacious alternatives. The advent of Aurlumyn represents a paradigm shift in frostbite management, offering a ray of hope to patients and healthcare providers alike.
Dr. Peter Hackett, a professor of medicine at the University of Colorado Anschutz Medical Campus who specializes in high-altitude and wilderness medicine, told CNN. “It’s a game-changer, in my opinion. It’s a major step forward in frostbite treatment in the United States.”
While the journey to FDA approval may have culminated on Valentine’s Day, the legacy of iloprost extends far beyond US borders. Countries such as Canada, Nepal, and various European nations have long recognized the therapeutic potential of iloprost in treating frostbite, attesting to its safety and efficacy even when administered days after the onset of symptoms.
Clinical trials have yielded promising results, with participants receiving iloprost demonstrating a stark reduction in the need for amputation compared to those treated solely with conventional medications.