Cancer claims hundreds of thousands of lives each year. People oftentimes assume that once they’ve been diagnosed with the disease, their years are already counted.
While this may be true for many, scientists are constantly hard at work to find the perfect cure. They have seen hope over the horizon, and for those who have been diagnosed with a type of pancreatic cancer that’s difficult to treat, there may be a new method that will help extend, and even save, their lives.
In fact, these researchers have discovered a new drug combination that may save their lives. Hope is just over the horizon, especially for those who have been diagnosed with pancreatic cancers that carry a KRAS wild-type mutation.The results seen in the new study are considered a significant advance for people with KRAS wild-type pancreatic cancer. This is a rare disease that needed more focus in research.
What the researchers did was add another type of drug to the current treatment regimen. There was a specific mix for this rare and particularly difficult to treat pancreatic cancer. The cancer, as mentioned earlier, is called KRAS wild-type. The new mix may be able to prolong life, according to new research presented June 3 at the American Society of Clinical Oncology (ASCO) annual meeting in Chicago.
There is an estimated 62,210 people in the United States that are said to be diagnosed with pancreatic cancer just this year. Sadly, many of the diagnosed cases will be at the advanced stage. With the available therapies prescribed by even the best in the field can only lead to a median overall survival benefit of just around six to eight months.
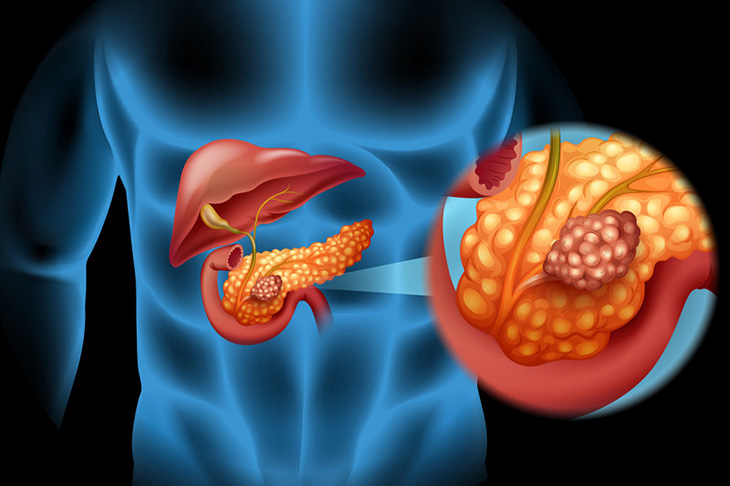
As for the mutations in the KRAS gene, these occur in between 70 to 90 percent of pancreatic ductal adenocarcinomas, and of them, KRAS wild-type is a distinct subtype. As for the five-year survival rate for this subtype, it is unfortunately less than 10 percent, which is a very low number.
The findings of the recent study made was generated by a phase 3 study of 92 patients in China. They discovered how those who became recipients of nimotuzumab in addition to the standard treatment for the disease, gemcitabine (Gemzar), survived for 10.9 months. They compared this with 8.5 months for those who received only gemcitabine.
Nimotuzumab is a type of therapy known as a monoclonal antibody. This came about from a collaboration between China and Cuba. As for now, the combination has not yet been approved by the FDA.
The dual-drug approach came about in a one-year survival rate of 43.6 percent, as compared to 13.9 percent among those who received gemcitabine alone. As for the three-year respective survival rate, it was 13.9 percent and 2.7 percent, respectively.
There were also patients who were not required to get surgery to take out the blockage of the pancreatic bile duct. The numbers came out even better for them, particularly on the treatment that they came up with. They compared the numbers to those who needed to undergo the said procedure. As for the progression-free period, which is the time wherein the disease did not progress, for those without a history of biliary surgery was 5.5 months for those who had gotten the combination treatment. This was against the 3.4 months in just the gemcitabine group.
Cathy Eng, MD, co-leader of VICC Gastrointestinal Cancer Research Program at Vanderbilt University, praised the research that was made for taking on a condition that, she noted, was “rarely investigated prospectively because … it represents less than 10 percent of all pancreatic cancer patients.”
The study offers a “potential treatment option for patients with advanced pancreatic cancer for whom current standard-of-care dual chemotherapy options are not feasible and single-agent gemcitabine is being considered,” said Arif Kamal, MD. He is the chief patient officer at the American Cancer Society. It must be noted that he was not involved in the research in any way.
He shared his thoughts on this and urged for researchers to conduct larger studies to evaluate the “toxicity profile and quality of life benefits” needed to fully know the role of nimotuzumab played in managing this difficult form of pancreatic cancer.