Ever since the term COVID-19 became a household name in January of 2020, scientists have been scrambling to find a cure. While laboratories in almost every corner of the globe managed to create vaccines in record time, some in as little as one year, while others can take anywhere between seven to ten years.
But vaccines aren’t the only type of medicine that scientists have rushed to develop to halt the pandemic. Another groundbreaking treatment is on the verge of a breakthrough, and it comes in nasal spray form. The hope is that this could possibly protect people from not just one, but all variants of COVID, as it advances to human trials from its successful lab testing.
The researchers at Northwestern University and the University of Washington share that the new spray happens to have “potent” proteins that work to target the weak spots of the virus, which in turn will prevent it from infecting the body.
Other treatments, such as the vaccines, have actually become less effective at fighting off COVID and the new variants that have since evolved from the first one.
Meanwhile, there were a number of antibody treatments that were stopped last month within the United States since they failed to protect people against the BA.2 omicron sub-variants, which also required complex refrigerated supply chains to continue.
In the April 12, 2022 journal Science Translational Medicine, it shares how this new and simpler solution against the deadly virus has been given approval to move forward to human clinical trials after showing great success in mice tests. These nasal sprays are also being tested in a number of different research institutes in Israel and England as well.
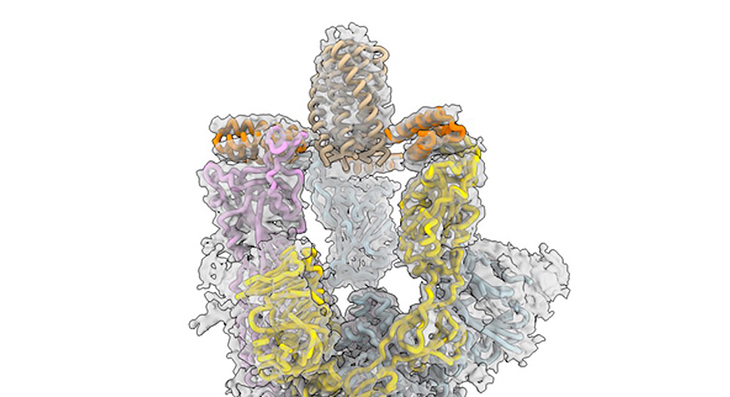
These sprays were created by American scientists who first used supercomputers to design proteins with the ability to stick to vulnerable sites on the surface of the novel coronavirus, precisely targeting the spike protein. The work was reported back in 2020 in the Science journal.
In this new work, the team reengineered the proteins, which are called minibinders, and made them even more potent. Rather than only targeting one site of the virus’ infectious machinery, these minibinders managed to bind to three sites, all at the same time, making the drug less likely to detach, they explain.
University of Washington School of Medicine’s Professor Michael Jewett shared that ‘SARS-CoV-2’s spike protein has three binding domains, but common antibody therapies may only block one’ it would seem.
Professor Jewett said, “Our minibinders sit on top of the spike protein like a tripod and block all three. The interaction between the spike protein and our antiviral is among the tightest interactions known in biology. When we put the spike protein and our antiviral therapeutic in a test tube together for a week, they stayed connected and never fell apart.”
The researchers also share that this nasal spray treatment managed to lessen the COVID symptoms outright, while also preventing infections from the get go when they tested it on mice.
Moreover, the proteins stopped COVID from binding to what’s known as the ACE2 receptor, which happens to be the entry point for the virus to infect the body. The virus cannot infect the body without first binding to the receptor, and this is why they believe that this new treatment will work against any new variant that might develop.
“To enter the body, the spike protein and ACE2 receptor engage in a handshake. Our antiviral blocks this handshake and, as a bonus, has resistance to viral escape,” added Professor Jewett.
These virus-hunting proteins may just give an alternative to the vaccines, which need a healthcare professional to administer each dose, unlike the nasal spray.
They can also be produced on a large scale in microorganisms such as E. coli, which makes them way more cost-effective to produce, while also being stable in high levels of heat. There is also a bigger chance that this treatment may be self-administered with a one-time nasal spray.
The research group hopes that for as long as their human clinical trials are successful, this particular treatment will be made available in pharmacies all over the world.