For the first time, the U.S. Food and Drug Administration approved the Janus kinase (JAK) inhibitor baricitinib as a treatment for those who suffer from severe alopecia areata. This is a painful and disfiguring skin disease for many. There is finally hope for those who want the use of something effective.
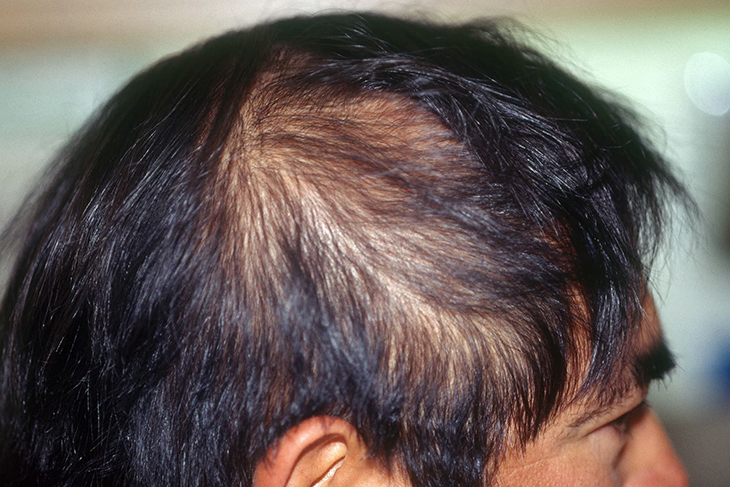
This first ever approved treatment for alopecia areata may be the solution to an autoimmune disorder that affects around 7 million people in the U.S. alone. Alopecia is a disfiguring disease where the body’s immune system starts to attack hair follicles. As a result, patients may find patchy or spots on their scalp, eyebrows, eyelashes, facial hair, and body hair.
Dr. Brett King is an associate professor of dermatology at Yale Medical School. He had worked with the reputable pharmaceutical company Eli Lilly and Company. With the collaboration, they went on to conduct a series of clinical trials with the new medicine: a once-a-day pill known as Olumiant.
In the trials they did, Olumiant helped one in three patients with severe alopecia areata. They were able to regrow their hair. It must be noted that almost half of the patients had no scalp hair at the very beginning. This means that they were able to cover more than 80 percent of the scalp. Improvements were also seen for those concerning eyebrow or eyelash hair loss.
In the last ten years, King has conducted innovative research making use of JAK inhibitors. These were primarily designed to treat rheumatoid arthritis and certain blood disorders. These inhibitors were then used to treat a variety of intractable skin diseases such as eczema, vitiligo, granuloma annulare, sarcoidosis, and erosive lichen planus.
“Until now, there have been no FDA-approved treatments for alopecia areata,” King said, “and the medicines that have been used in the past to treat severe cases of alopecia areata are largely ineffective. There is, however, lots of data to show that a relatively new class of medicines called JAK inhibitors work for the treatment of severe alopecia areata. Patient access to these medicines is extremely limited, though, because JAK inhibitors were not FDA-approved for this purpose. FDA approval will bring greater access, via insurance coverage, to patients.”
He said that the approval given by the DFA also had an added benefit. “When a medicine is approved for treatment of a disease, doctors feel more comfortable prescribing the medicine for that purpose. Therefore, FDA approval will empower and enable health care providers to treat patients with severe alopecia areata,” he explained.
He also recalled the first ever patient he treated. “He had almost no scalp hair, his eyebrows and eyelashes and facial hair were missing, and, in addition, he had red, scaly psoriasis plaques all over his body. It was in 2013… I explained to the patient that use of tofacitinib in him would be exploratory, and he agreed to try it…. Not long after he started taking tofacitinib, his hair started to grow. I published the results of his treatment not long after that and history was made, forever changing this disease,” King said. It has truly been revolutionary and the improvements have been witnessed.
The results of the trials done had been published in New England Journal of Medicine.