Bacteria In Sewers May Be The Answer To Finding Cures And Preventions For Diseases With Antimicrobial Resistance
As crazy as it sounds, our biggest enemy when it comes to health may also actually be our biggest ally. That’s because antimicrobial resistance, also called AMR, is aiding in the creation of “superbugs,” which is now threatening people’s health on a day to day basis. In fact, this may be the very reason why your flu now lasts way longer than it used to.
According to research, around 60% of the most common strains of bacteria that live in our societies are resistant to antibiotics in one way or another. This is why the Eliava Institute of Bacteriophages, Microbiology, and Virology in Tbilisi, Georgia is studying the effects of “phage therapy” on the treatment of AMR for years. In fact, their research goes back to when they were still part of the Soviet Union.
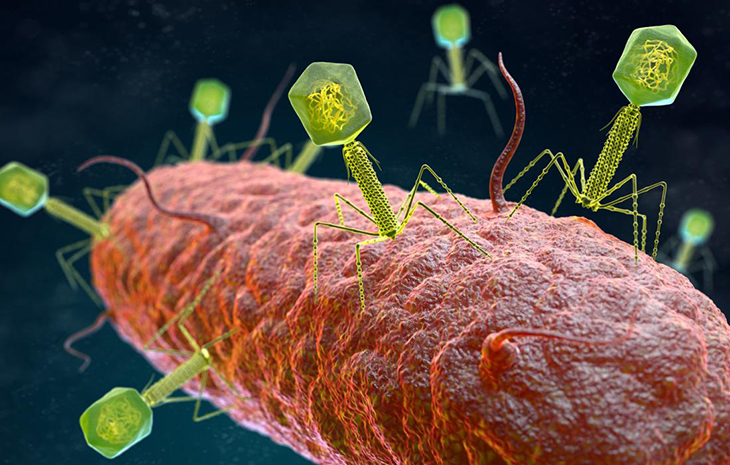
How phage therapy works is that is takes bacteriophages – a type of virus ‘that infects, replicates in and kills bacterial microbes’ – as a way to clear these AMR infections. The institute performed studies between 2014 and 2018 that resulted in over 95% improvement and recovery rate and no cases of side effects or complications after the application of the phage.
But, the challenge is in finding these tiny lifeforms in the first place.
According to a report in National Geographic, some virologists from Kenya that were digging through Nairobi’s sewage – an area where quadrillions of microbes are hard at work as they break down matter in the sewage systems. These extremely high amounts of bacteria eventually attract their predators, otherwise known as bacteriophages.
The virologists took some phages that they caught in the sewage plant and brought them to the Kenyan Medical Research Institute’s Department of Emerging Infectious Diseases (KEMRI). Here, they isolated some and tested them against two AMR strains of Klebsiella pneumonia and Pseudomonas aeruginosa, which are two of the most common AMR superbugs found in Kenya.
However, the virologists can’t know what it is that killed the bacteria since ‘phages are microscopic in relation to bacteria, which are microscopic to our eyes.’ Rather, the evidence of the death of bacteria is found while the sample is stored in deep freeze for investigation later on.
This type of research is considered much more cost-effective and faster than making new antibiotics, which tend to have a more substantial financial risk that’s normally imposed by government agencies, like the FDA, since it needs to go through a series of trials to test it efficacy. Most pharma companies stand to lose up to $10 billion or more even, if the drug makes it all the way to stage 3 trials but eventually fails anyway. Notably, there have been no new antibiotics developed, created and manufactured since 1980.
Moreover, another advantage for this type of research, mostly for the phage therapy in both African and Asian countries that don’t have their own domestic pharmaceutical industry, is that these bacteriophages usually are more effective when they live in the same environment as the AMR bacteria. They also discovered in the Kenya’s research institute that ‘phages from Georgia weren’t as effective as killing superbugs that evolved in Kenya.’
Plus, phages usually evolve and change to prey on one bacteria species, which means that when used on humans, they won’t necessarily harm the plethora of existing non-harmful, friendly microbes that are so important to the overall health and wellbeing of humans.
So far, the Kenyan team has identified at least 150 different phages, and within the US, there are already 60 clinical trials that are registered to test these phages for their safety and efficacy in treating AMR superbug infections. If they work, we may be looking at finally getting some new antibiotics on the market.